IFA
EUROIMMUN Indirect Immunofluorescence: simple and modern method
Principle of the test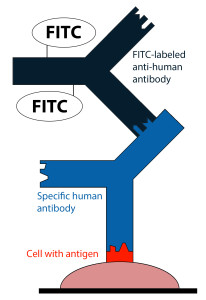
- For the determination of autoantibodies or antibodies against infectious agents, cells, tissue sections or purified, biochemically characterized substances are used as antigen substrates.
- If the sample is positive, specific antibodies in the diluted serum sample attach to the antigens coupled to a solid phase.
- In a second step, the attached antibodies are stained with fluorescein-labelled anti-human antibodies and visualized with the fluorescence microscope.
- Positive samples can be titrated in steps. The most suitable titration interval is provided by the dilution factor 3.162 (square root of 10). In this way, every second step represents in its denominator an integral power of 10 (1:10, 1:32, 1:100, 1:320, 1:1000, 1:3200, 1:10000 etc.).
Indirect immunofluorescence: a standardized technique for the determination of autoantibodies and antibodies against infectious agents
- High specificity: positive and negative samples produce a large difference in signal strength. Each bound antibody shows a typical fluorescence pattern depending on the location of the individual antigens.
- The entire antigen spectrum of the original substrate is available, thus allowing the detection of a large number of antibodies and achieving a higher detection rate.
- Immunofluorescence enables simultaneous detection of antibodies against several biochemically different antigens on one single biological substrate.
- The indirect immunofluorescence test is the analytical method of choice when it would be too difficult or too complicated to prepare the test antigens individually for enzyme immunoassays.
EUROIMMUN’s innovations for the standardization and modernization of indirect immunofluorescence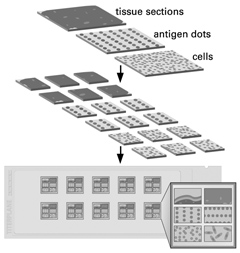
- Activation Technique: physically or chemically activated cover glasses are coated with cultured cells or tissue sections. Frozen tissue sections are fixed to the glass surface by covalent bonding, increasing adhesion more than 100 times and thus preventing the substrates from being detached.
- BIOCHIP Technology: cover glasses coated with biological substrates are cut into millimeter-sized fragments (BIOCHIPs) on a machine. This makes it possible to obtain ten or more first-class preparations of homogeneous quality per tissue section, in the case of cultured cell substrates even several thousands.
- BIOCHIP Mosaics™: using several BIOCHIPs coated with different substrates side by side on one and the same reaction field, antibodies against various organs or infectious agents can be investigated simultaneously. Detailed antibody profiles can thus be established with comparatively little effort, allowing the reciprocal determination of the results on different substrates.
- TITERPLANE™ Technique: samples or reagents are applied to the reaction fields of a reagent tray. The BIOCHIP Slides are then placed into the recesses of the reagent tray, where all BIOCHIPs come into contact with the fluids, and the individual reactions commence simultaneously. As the fluids are confined in a closed space, there is no need for the use of a conventional "humidity chamber".


